There are opportunities for medtech players to make the most of R&D spending, find partner André Rocha and coauthors. For example, since 2011, the average number of US Food and Drug Administration device approvals per billion dollars spent on R&D for medtechs has declined by an average of 9 percent per year. Companies could look to boost capabilities such as product management, design thinking, and systems engineering.
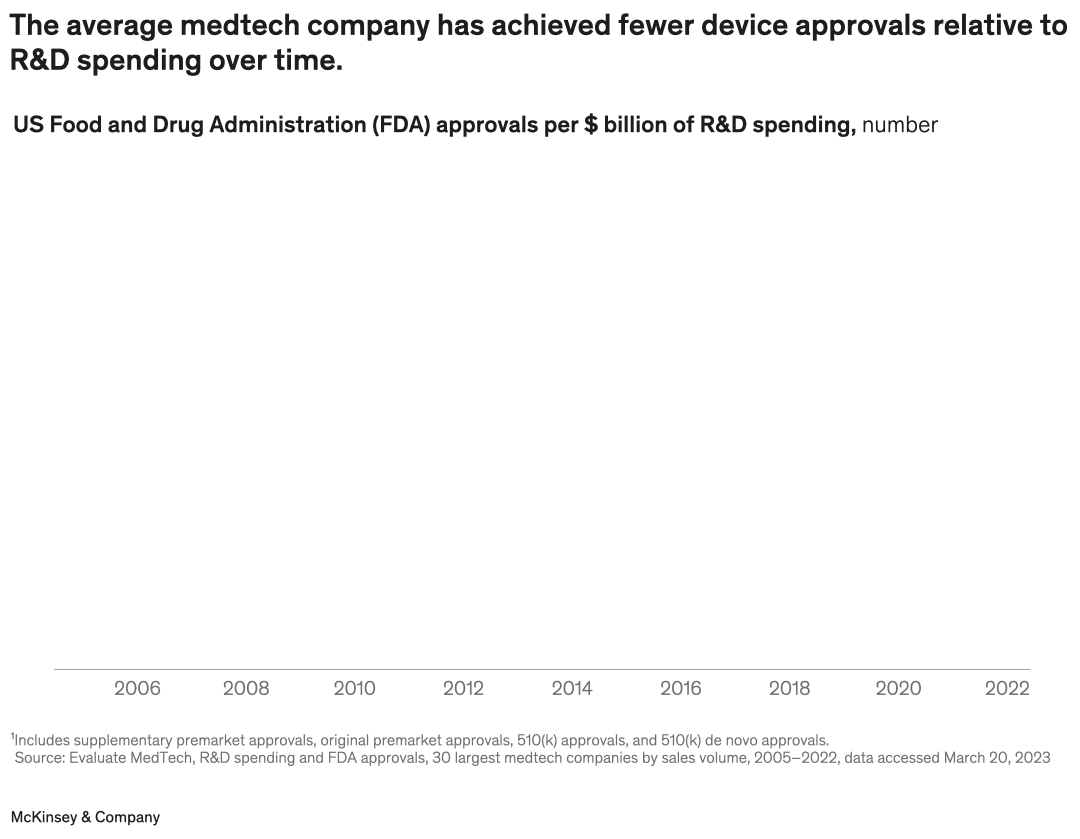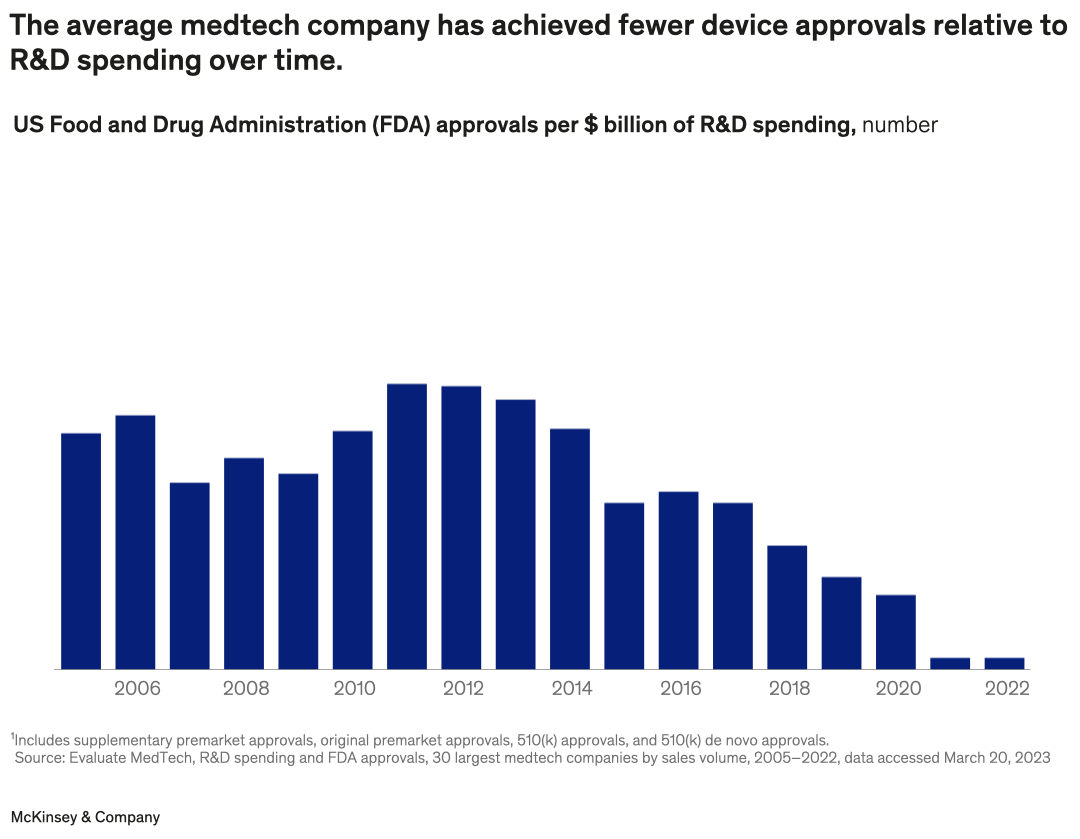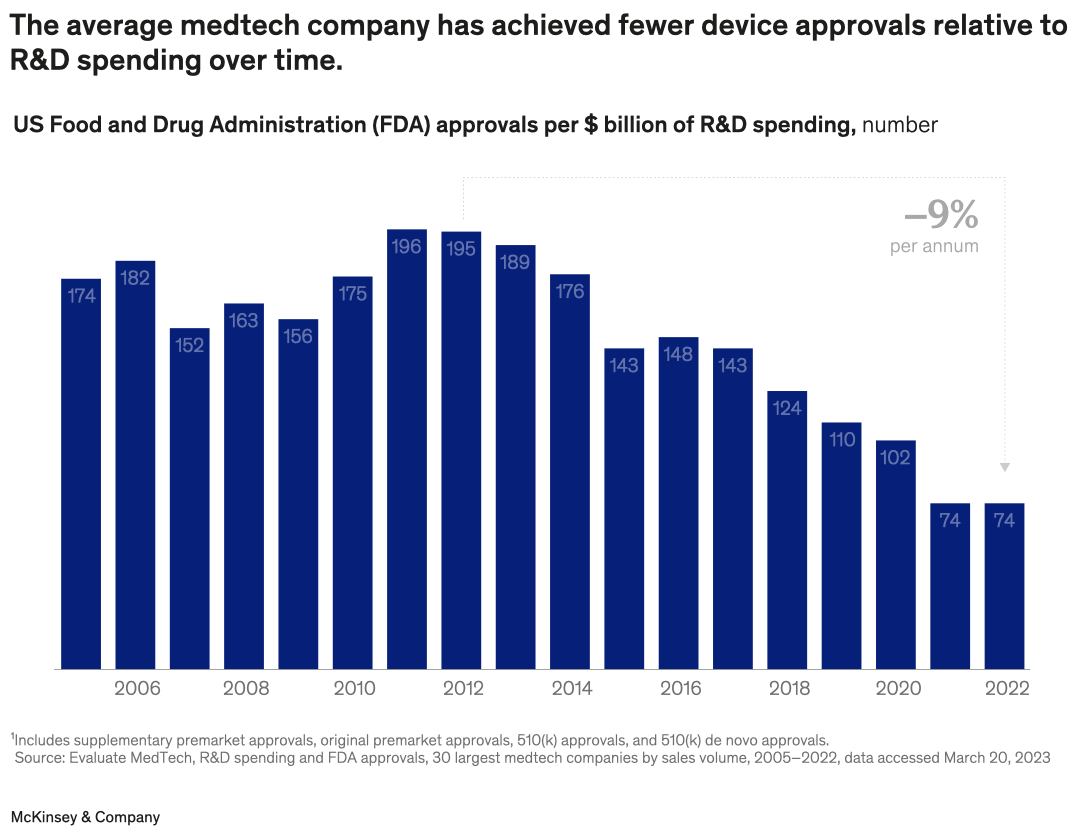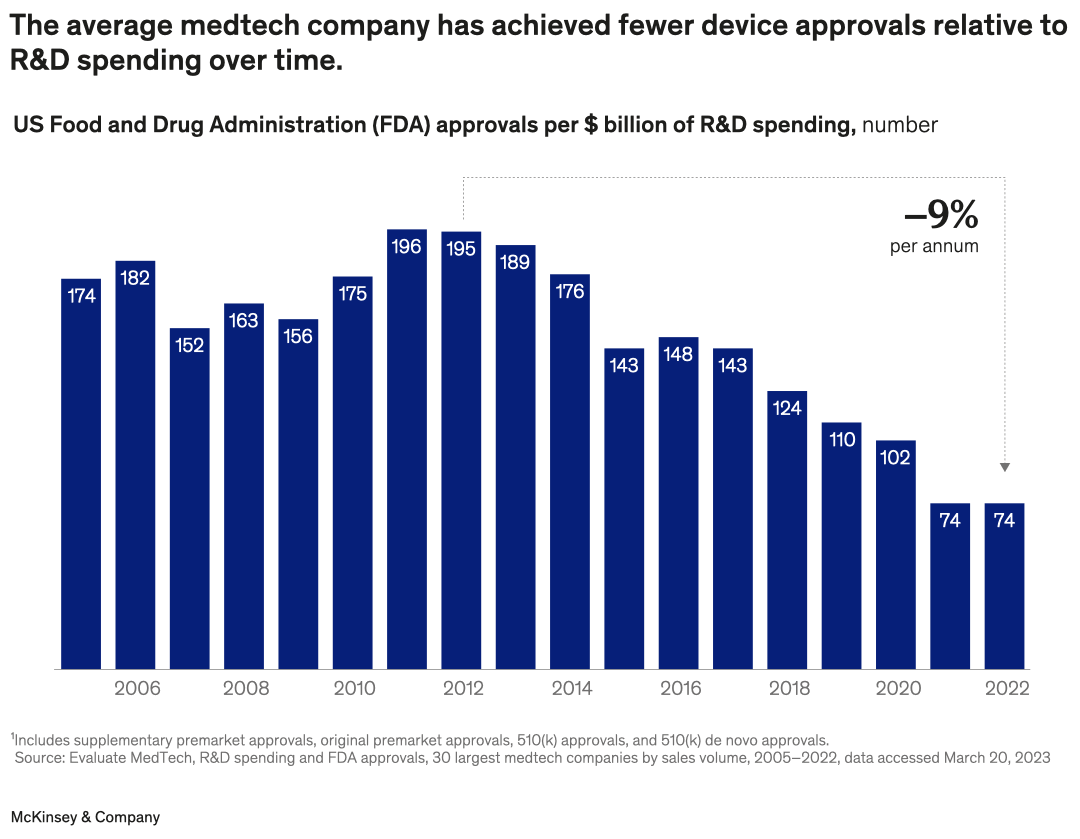
Image description:
A bar graph shows the number of US FDA approvals for medtech devices per $ billion of R&D spending in 2005–22. For the first decade, the number of approvals is between 152 and 196 per year. After 2014, the number begins to drop, until in 2021–22, 74 approvals were given, a –9% per year decline from 2012.
Footnote: Includes supplementary premarket approvals, original premarket approvals, 510(k) approvals, and 510(k) de novo approvals.
Source: Evaluate MedTech, R&D spending and FDA approvals, 30 largest medtech companies by sales volume, 2005–2022, data accessed Mar 20, 2023.
End of image description.
To read the report, see “Medtech Pulse: Thriving in the next decade,” September 15, 2023.